Project - Frontotemporal dementia and motor neurodegenerative syndromes
Disease area:
Neurology, Dementia, MND, FTD
Professor Matthew Kiernan, Associate Professor Susanna Park, Professor Cindy Lin, Professor Steve Vucic, Dr William Huynh, Dr Jaschelle Caga, Associate Professor Rebekah Ahmed, Dr Emma Devenney, Dr Colin Mahoney, Dr Sicong Tu, Dr Antonia Carroll, Sr Margie Zoing, Dr Hannah Timmins, Srestha Mazumder, Professor Michael Barnett, Professor Glenda Halliday
Abstract
Confirmation of diagnosis and the assessment of regional patterns of neurodegeneration require systematic neuropathology. At disease onset, focal pathology restricted to distinct brain areas is a feature of MND, FTD and related syndromes. Forefront Neurology have introduced a multidisciplinary clinical assessment for MND and FTD patients referred to Royal Prince Alfred Hospital (partner in NHMRC Advanced Health Research and Translation Centre, Sydney Health Partners) and the Brain and Mind Centre, University of Sydney. As such, all patients currently undergo standard clinical, electrodiagnostic, neurophysiological, respiratory and functional grading assessments, a comprehensive core neuropsychological assessment, in addition to a range of behavioural assessments and neuroimaging. Bloods are taken and stored for DNA and biomarker testing. Clinical phenotyping is refined over time through ongoing clinical assessments undertaken by an extended multidisciplinary clinical and research team. The ultimate goal for neurodegenerative research remains to develop a platform for therapeutic intervention with disease-modifying therapies. This requires an understanding of the pathogenesis of the protein abnormality to be treated, as well as the ability to clinically identify the patient with this protein abnormality and specific clinical phenotype.
Challenges within the field
1. To translate research findings in FTD/MND into diagnostic and phenotypic tools that can be utilised in tertiary clinics and broader healthcare settings, nationally and internationally.
2. To develop interventions that alter the traditional disease trajectory, to better understand the cognitive and behavioural changes in early MND and FTD.
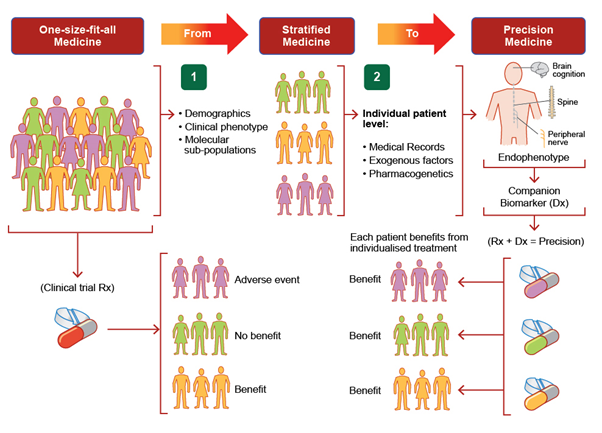
Disease heterogeneity suggests that a precision medicine paradigm will be required to realise effective therapy, enabled through incorporation of phenotypic and genotypic information to improve outcomes for individual patients
Key Findings
Recent progress has promoted the transition of neurodegenerative disease to a precision medicine paradigm, enabled through the incorporation of phenotypic and genotypic data to better guide therapy and thereby outcomes for individual patients. Such prediction promotes better targeted therapies for neurodegenerative diseases. Recent progress with precision medicine based on genetically-targeted therapies is anticipated to have a significant impact on the natural history of disease.
Research Objectives:
- Diagnosis and treatment of Neurological Disease, with specific focus on Frontotemporal Dementia and Motor Neurodegeration Syndrome Program.
- Investigation of disease pathophysiology and treatment strategies for neurological disorders.
- Optimise the translation of research findings into improved healthcare practice and policy.
Research Project Description
A critical feature leading to successful therapy development for neurodegenerative syndromes will be to facilitate inclusion of appropriate mechanistic outcome measures into clinical trial programs. Currently there is a disconnect between efforts to develop sensitive and specific mechanistic outcome measures and clinical trial protocol development. Common mechanisms associated with both metabolic dysfunction and neurodegeneration include oxidative stress, inflammation and vascular dysfunction. While there is limited in vivo evidence to suggest that metabolic mechanisms may enhance neurodegeneration, there is emerging data on the metabolic variability associated with MND/FTD that suggests a spectrum of phenotypic metabolic change that may be developed as models. In the least, metabolic changes contribute to patient disability including the muscle wasting, a significant prognostic factor.
