I am a final year Ph.D candidate at the Brain and Mind Centre. I use a variety of histological and molecular biological techniques to explore neurotransmitter changes arising from the brainstem in Parkinson’s disease. I am particularly interested in linking these changes to upstream neurotransmitter levels in forebrain regions alongside non-motor clinical manifestations in Parkinson’s disease.
Forefront Group: Prof. Glenda Halliday’s Neurodegenerative Lab
Supervisors:
Professor Glenda Halliday, Dr YuHong Fu, Dr James M. Shine
Neurodegeneration of interest:
Parkinson’s disease, dementia, cognition, movement disorders.
Expertise:
- Neuropathology
- Molecular biology
Affiliate Organisations
University of Sydney
Specific Skills:
- Immunohistochemistry
- Immunofluorescence
- Confocal microscopy
Project - The Staging of Neurotransmitter System Deterioration in the Non-Motor Symptoms of Parkinson’s Disease
Disease area:
PD, PDD, ILBD
Research Project Description
My project aims to understand the holistic pattern of deterioration of important neurotransmitter systems alongside the development of depression and dementia in Parkinson’s disease. This objects to give greater insight into the pathogenic associations to these symptoms in neurodegenerative diseases. To date, such comprehensive information is missing. We hypothesise that the degeneration of neurotransmitter systems occurs specifically in PD patients with depression and dementia and differentiates these patients from cases without these symptoms and those with overlapping neurodegenerative diseases (Idiopathic Lewy Body Disease, Alzheimer’s disease, Dementia with Lewy Body). My aims are:
- To evaluate the levels of neurotransmitter and pathology relevant proteins in standardised brain regions.
- To examine the degree of cell loss by quantifying the numbers of neurotransmitter-generating neurons in the main neurotransmitter nuclei.
- To evaluate the specific pathological stages of the different pathoproteins.
- To determine the associations between the timing of the onset of depression and dementia, pathological disease stages, neurotransmitter-generating cell loss and neurotransmitter and pathoprotein levels.
Parkinson’s disease (PD) is characterised by motor symptoms due to the loss of neurons making the neurotransmitter dopamine. PD patients also present non-motor symptoms that are prevalent throughout the disease course due to more widespread neurodegeneration. Among the non-motor symptoms, the prevalence rates of early depression and late dementia are the highest, 50% and 60%, respectively, and negatively impact on disease progression and mortality. Degeneration of serotonergic, cholinergic, noradrenergic, and dopaminergic systems have all been associated with the non-motor symptoms of PD, but the extent and timing of degeneration in these neurotransmitter systems with respect to the development of depression and dementia in PD remain unknown. This project aims to understand the staging of degeneration in these neurotransmitter systems in line with the occurrence of depression and dementia in PD. To reveal these clinicopathological associations, the pathological changes of these neurotransmitter systems in the post-mortem brain regions of PD patients with and without depression and dementia will be examined and compared to results from those of diseases that were often clinically misdiagnosed with PD. This data will provide basic knowledge of symptom-associated degeneration of therapeutically targetable neurotransmitters to ultimately improve disease management and promote patients’ life quality.
For Aim 1: Western blotting and/or ELISA will be used to screen neurotransmitter and pathology relevant proteins with altered expression level in diseased conditions in the neurotransmitter target brain regions. Quantitation of western blot images will be captured with Bio-Rad Chemidoc and assessed with Image Lab software (Bio-Rad) and Image J (VER 1.46, National Institute of Health). ELISA results will be read by a plate reader.
For Aim 2: Paraffin sections will be cut at 8um on a Leica microtome for immunohistochemistry and Nissl counter staining. Stains will be performed in brain regions containing neurons producing neurotransmitters. Neurotransmitter-generating neurons will be identified using antibodies against rate limiting enzymes. In addition, other markers assisting in identifying subgroups of neurotransmitter neurons will be also applied. Positive neurons will be counted with the assistance of ImageJ cell counter plugin tool (VER 1.46, National Institute of Health) on images scanned using an Olympus VS120 slide scanner set at 20x objective.
For Aim 3: The severity of three types of pathology will be determined in the same brain regions of objects 1 and 2 using IHC with antibodies against alpha-synuclein, phosphorylated-tau, and amyloid-β. Disease staging will be assessed using current neuropathological diagnostic criteria. Double or triple labelling of immunofluorescence staining will be examined on a Nikon C2 confocal microscope, scanned with Olympus VS120 slide scanner, and the images analysed with Image J (VER 1.46, National Institute of Health) software to determine the proportion of double or triple labelled cells.
For Aim 4: Clinicopathological stages and associations will be determined using standardised pattern assessment and statistical evaluations.
Infographic / Medical Diagram / Scientific Diagram / Picture
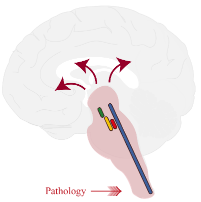
Brainstem dopaminergic (green), noradrenergic (red), serotonergic (blue), and cholinergic (yellow) nuclei. Figure models Braak stage 3 showing that the pathology affecting brainstem will progress to the wide brain regions.