Project - “Cerebellar contributions to cognitive changes in frontotemporal dementias”
Research Project Abstract
Mounting evidence indicates that the impact of cerebellar damage extends beyond motor function, and also affects cognition. FTD is associated with heterogeneous patterns of cortical atrophy, primarily affecting the frontal and/or temporal lobes. Previous research has also found cerebellar changes in FTD. The cognitive role of the cerebellum in FTD, however, remains unclear. This project (2015-2019), therefore, examines cerebellar contributions to cognitive changes using a combination of behavioural and neuroimaging approaches in the three main FTD subtypes, each with distinct clinical cognitive profiles: behavioural-variant FTD (bvFTD), semantic dementia (SD), and progressive nonfluent aphasia (PNFA). The results from this project demonstrated that the cerebellum is affected in all the three FTD subtypes, with syndrome-specific patterns of cerebellar changes across subtypes. These cerebellar changes are associated with cognitive performance in FTD. Importantly, cerebellar atrophy progresses over time and is associated with cognitive decline with disease progression. These findings extend the understanding of the cerebellum and point to its involvement across an array of processes beyond the domain of motor function. From a clinical perspective, these novel findings bring new insights into the mechanisms mediating FTD symptomatology, and have important implications for disease management, intervention and wellbeing for both patients and carers.
Project with a disease tag
FTD, FTD-MND
Challenges within the field
Frontotemporal dementia (FTD) is a leading cause of dementia in people before the age of 65. The progressive decline in behaviour and cognition in FTD is primarily associated with brain changes in the frontal and/or anterior temporal lobes. The progressive decline in behaviour and cognition in FTD is primarily associated with brain changes in the frontal and/or anterior temporal lobes. The extent of cerebellar structural abnormalities, their evolution with disease progression, and their relations to cognitive functioning remain poorly understood.
In this project, we combined multimodal neuroimaging techniques and comprehensive neuropsychological assessments to investigate the role of the cerebellum in FTD.
Research Project Description
In the context of the existing evidence of the role of the cerebellum in FTD, this project had five studies:
- i. Review the available data in the neuroimaging literature and synthesise evidence for possible role of the cerebellum in cognition in FTD. (ALE meta-analysis)
- ii. Establish patterns of cerebellar grey matter atrophy in the three main FTD subtypes on structural MRI and identify their relation to profiles of cognitive deficits. (VBM analysis)
- iii. Examine whether connectivity between the cerebellum and the cerebrum is affected in subtypes of FTD and determine its contributions to cognitive deficits. (TBSS analysis)
- iv. Elucidate disease-specific progressive cerebellar grey matter changes in FTD subtypes and investigate the relations between progression of cerebellar atrophy and profiles of cognitive decline. (Longitudinal VBM analysis)
- v. Characterise patterns of cerebellar grey matter atrophy and the underlying neural correlates of cognitive deficits in FTD patients with C9orf72 hexanucleotide repeat expansion in comparison with non-carriers. (VBM analysis)
Research Objectives
- To establish the patterns of cerebellar changes in FTD;
- To examine the degree of neuroanatomical variability between individuals with a genetic abnormality (i.e., chromosome 9 open reading frame 72 gene repeat expansion) and non-carriers;
- To investigate how cerebellar changes contribute to the clinical profile and cognitive deficits observed in FTD.
Key Publications from this project
- Yu Chen, Fiona Kumfor, Ramon Landin-Romero, et al. Cerebellar structural connectivity and contributions to cognition in frontotemporal dementias. Cortex, 2019, DOI 10.1016/j.cortex.2020.04.013. (In press)
- Yu Chen, Fiona Kumfor, Ramon Landin-Romero, et al. The cerebellum in frontotemporal dementia: a meta-analysis of neuroimaging studies. Neuropsychology Review, 2019, 29 (4): 450-464.
- Yu Chen, Fiona Kumfor, Ramon Landin-Romero, et al. Cerebellar atrophy and its contribution to cognition in frontotemporal dementias. Annals of Neurology, 2018, 84 (1):98-109.
- Yu Chen, Fiona Kumfor, Ramon Landin-Romero, et al. Cerebellar atrophy and its contribution to cognition in frontotemporal dementias. Annals of Neurology, 2018, 84 (1):98-109.
Key Findings
The objectives of this project were to identify the changes in the cerebellum in FTD and their impact on cognition and behaviour, using a combination of clinical and cognitive measures and structural neuroimaging techniques. Investigations from the five studies included in this project provide convergent evidence of cerebellar involvement in the main subtypes of FTD with specific contributions to cognitive changes, resulting in an improved understanding of the mechanisms mediating FTD symptomatology.
The meta-analysis identified extensive evidence of cerebellar involvement in FTD from previous neuroimaging studies. We further confirmed these findings directly by testing these ideas in patient populations and uncovered patterns of cerebellar grey and white matter changes and their associations with cognitive dysfunctions across the FTD subtypes. The longitudinal study revealed the progression of cerebellar degeneration over time, and which is correlated with disease progression and cognitive decline in the three FTD subtypes. Finally, we compared cerebellar involvement between non-carriers and genetic FTD cases (i.e., C9orf72 expansion carriers).
This project has provided important new insights regarding cerebellar dysfunction and its role in the origin of cognitive impairments in FTD syndromes. The findings challenge the prevailing notion about the role of the cerebellum and extend its involvement across an array of cognitive processes beyond the domain of motor function. In this way, the cerebrum can no longer be viewed as the sole driver of thinking and behaviours, and instead, we must adopt an integrated approach emphasising cerebro-cerebellar interactions in the service of complex cognitive functions. The approach implemented in this project demonstrates that studying cerebellar functions in patients with dementia can further the understanding of cerebellum-cognition relationships in the context of brain network disruption. We hope that identification of the nature of cerebellar changes and its impact on cognitive function in FTD may have important implications for the characterisation and management of these patients.
Infographic / Medical Diagram / Scientific Diagram / Picture
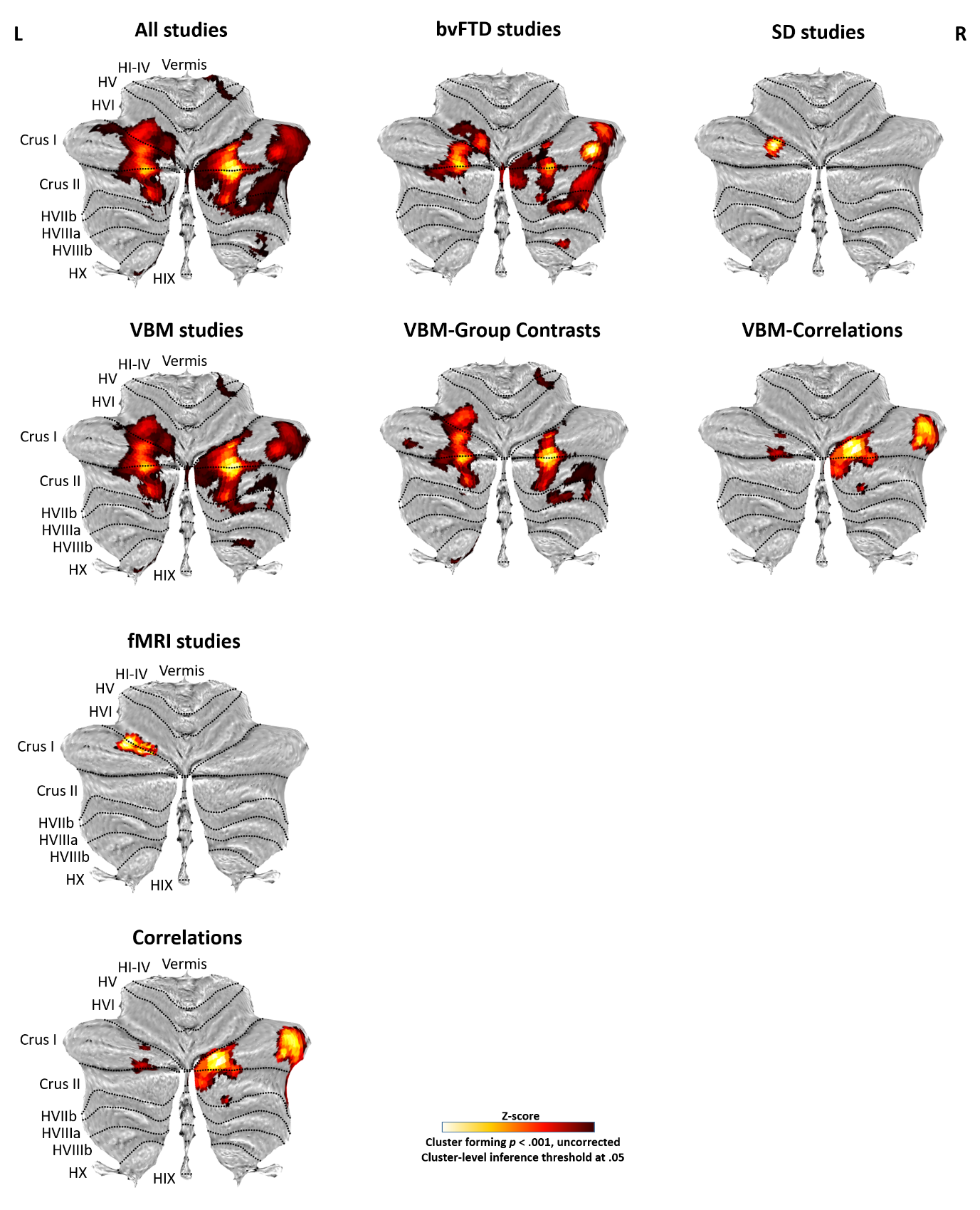
Figure 1. Cerebellar involvement in FTD (combining both whole-brain and region-of-interest studies). Cerebellar flatmaps of significant clusters are colour coded (cluster forming threshold p < .001 uncorrected, cluster-level inference threshold at 0.05). VBM = voxel-based morphometry; fMRI = functional magnetic resonance imaging; bvFTD = behavioural-variant frontotemporal dementia; SD = semantic dementia.
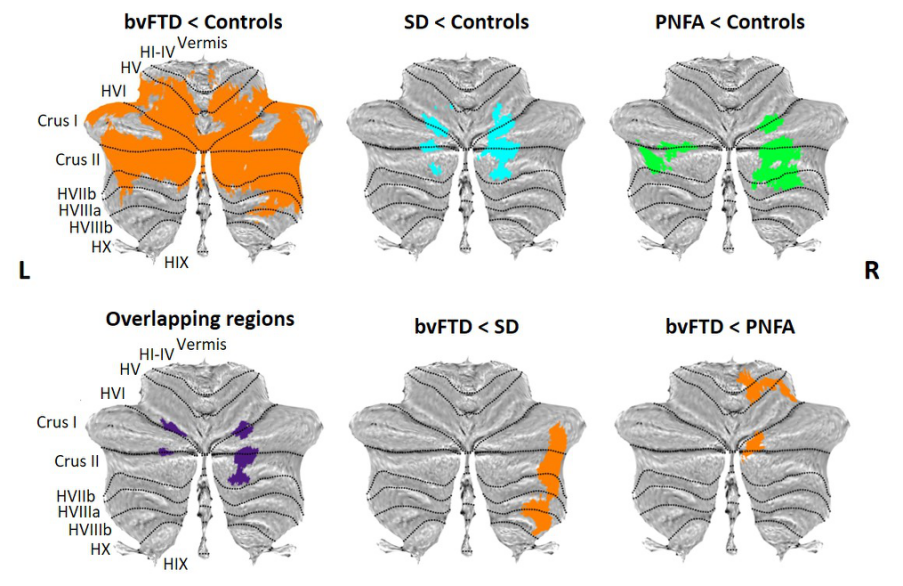
Figure 2. Voxel-based morphometry analyses showing regions of decreased cerebellar grey matter integrity in contrasts between patient groups and Controls, contrasts between disease groups, and the overlapping regions among the contrasts between patient groups and Controls. Coloured voxels show regions that were significant in the analyses at the threshold of p < .001 (uncorrected for multiple comparisons) with a cluster threshold of 100 contiguous voxels. bvFTD = behavioural-variant frontotemporal dementia; SD = semantic dementia; PNFA = progressive nonfluent aphasia.
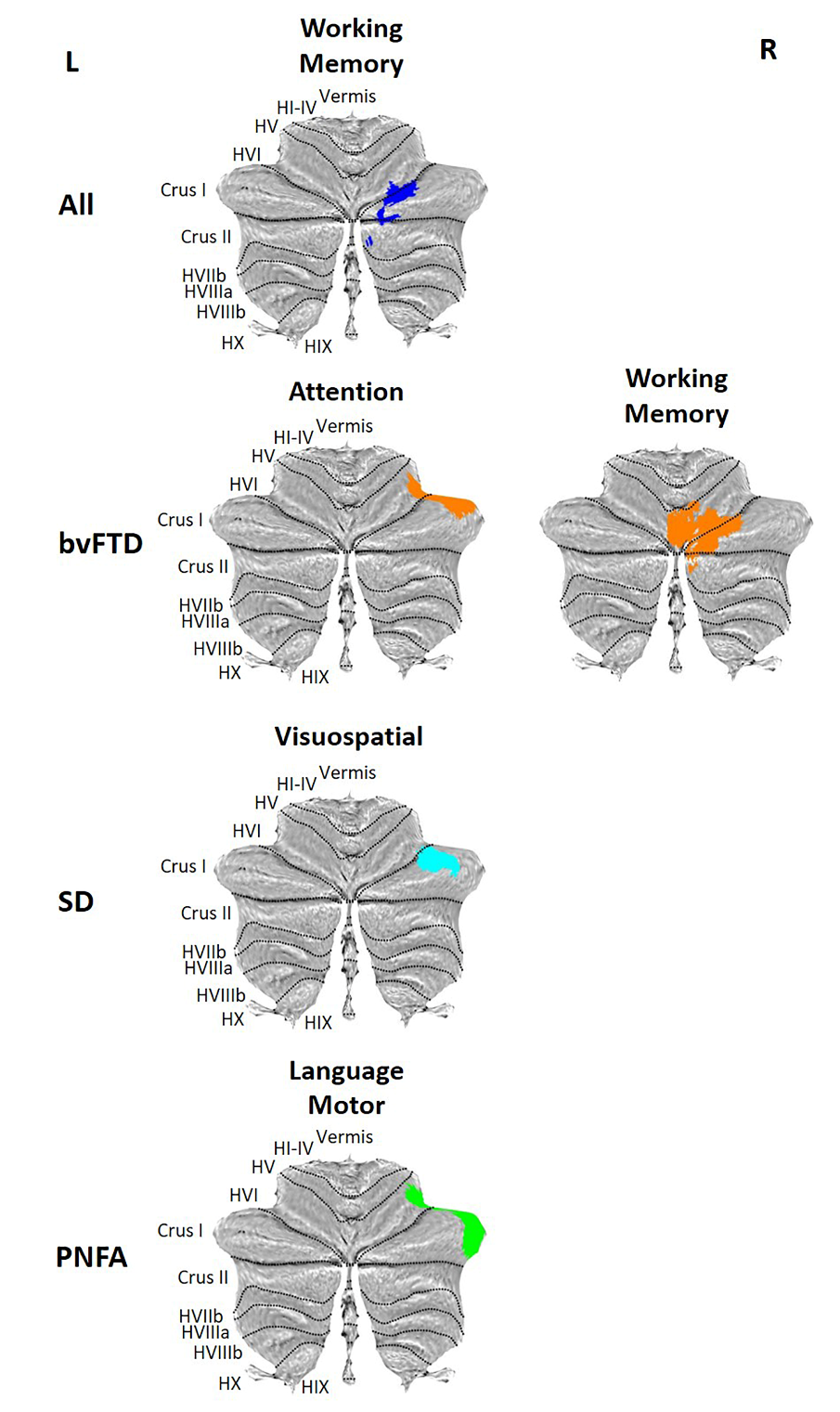
Figure 3. Voxel-based morphometry results showing regions that correlate with neuropsychological performance after covarying for cerebral atrophy. Coloured voxels show regions that were significant in the analyses at the threshold of p < .001 (uncorrected), with a cluster threshold of 100 contiguous voxels.
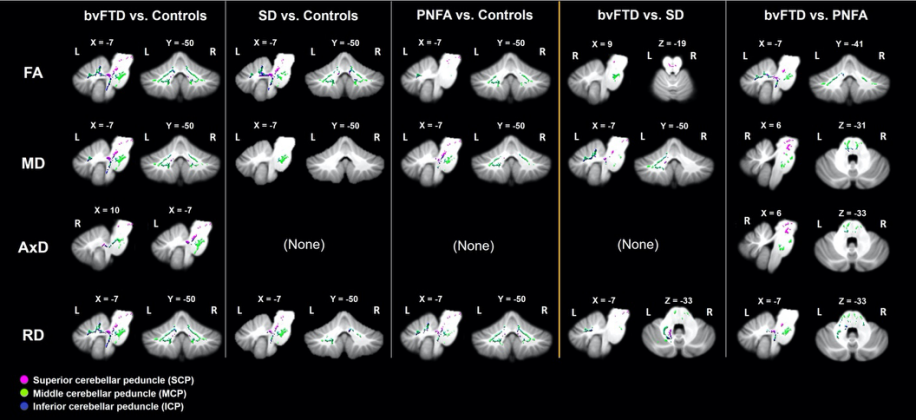
Figure 4. Tract-based spatial statistics showing regions of cerebellar white matter changes (reduced FA, increased MD, AxD and RD) in contrasts between groups. Coloured voxels show regions that were significant in the analyses at the threshold of p < .05, family wise error corrected for multiple comparisons, with a cluster extent threshold of 100 contiguous voxels. bvFTD = behavioural variant frontotemporal dementia; SD = semantic dementia; PNFA = progressive nonfluent aphasia; FA = fractional anisotropy; MD = mean diffusivity; AxD = longitudinal (axial) diffusivity; RD = radial diffusivity.
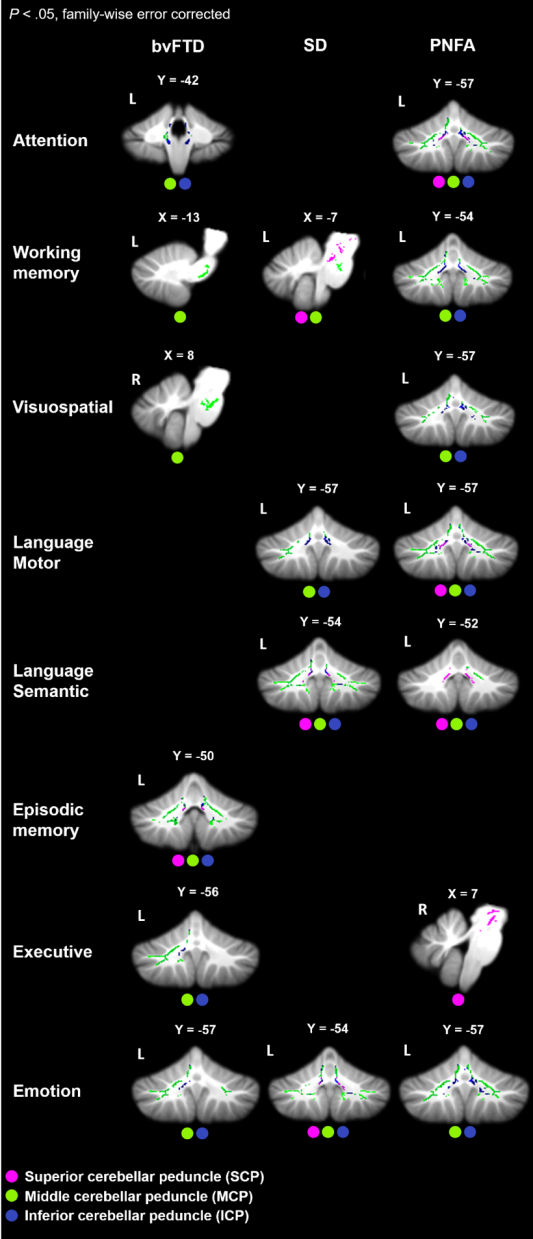
Figure 5. Correlations between fractional anisotropy values and neuropsychological performance in each patient groups and Controls combined (p < .05, family-wise error corrected for multiple comparisons). The coloured dots indicate the affected cerebellar peduncles.
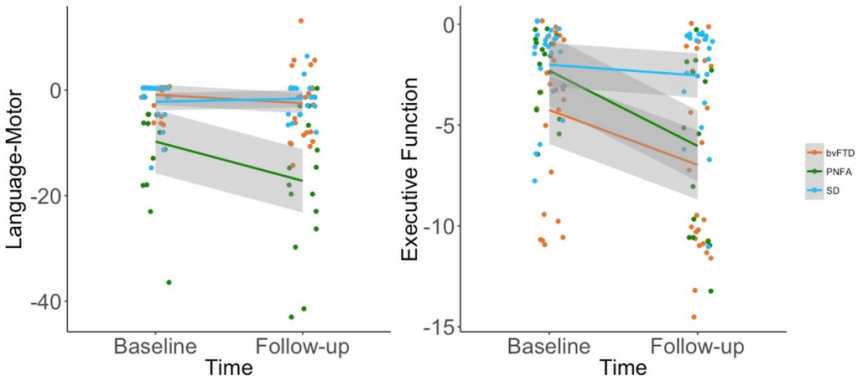
Figure 6. Longitudinal performance on the behavioural variables with significant Time × Diagnosis interaction. bvFTD = behavioural-variant frontotemporal dementia; PNFA = progressive nonfluent aphasia; SD = semantic dementia.
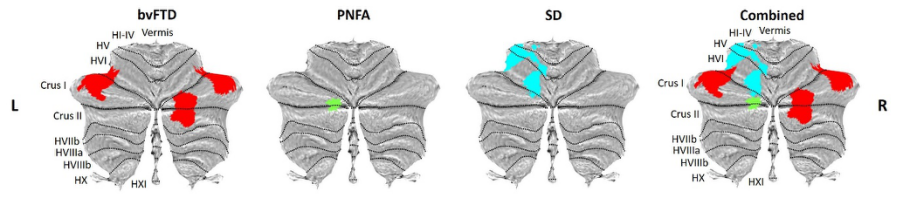
Figure 7. Longitudinal cerebellar grey matter changes according to diagnostic group. Coloured voxels show regions that were significant in the analyses at the threshold of p value < .05 corrected for multiple comparisons via Family-Wise Error (FWE) correction. bvFTD = behavioural-variant frontotemporal dementia; PNFA = progressive nonfluent aphasia; SD = semantic dementia.
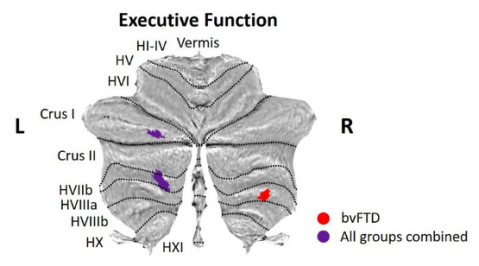
Figure 8. Associations between longitudinal cerebellar grey matter changes and cognitive progression. Coloured voxels show regions that were significant in the analyses at the threshold of p value < .05 corrected for multiple comparisons via Family-Wise Error (FWE) correction, with a cluster threshold of 50 contiguous voxels.
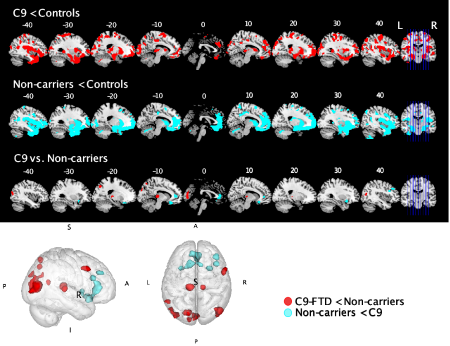
Figure 9. Whole brain voxel-based morphometry analyses showing regions of decreased grey matter intensity in contrasts between patient groups and controls. Coloured voxels show regions that were significant in the analyses at the threshold of p < .001 (uncorrected) with a cluster threshold of 50 contiguous voxels. The bottom row of brain slices and the 3D brain show direct contrasts between patient groups. Voxels in red show regions that are more affected in the C9orf72 positive group than the non-carrier group. Voxels in cyan show regions that are more affected in the non-carrier group than the C9orf72 positive group. C9 = C9orf72 positive group.
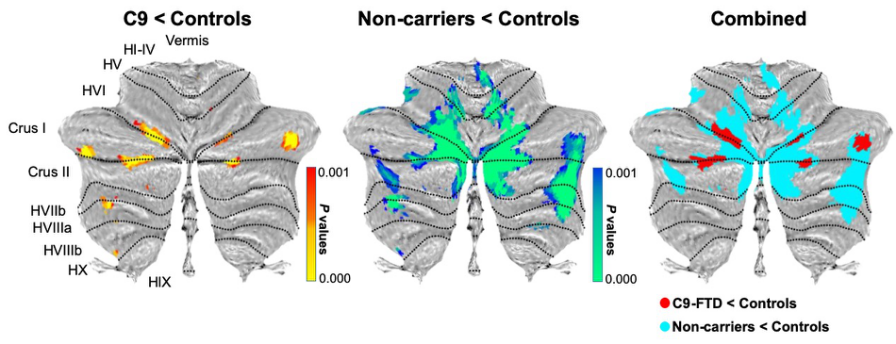
Figure 10. Voxel-based morphometry analyses showing regions of decreased cerebellar grey matter density in contrasts between patient groups and controls. The gradient coloured voxels show regions that were significant in the analyses at the threshold of p < .001 (uncorrected for multiple comparisons). Atrophy maps of each contrast between patient groups and controls are colour coded in the overlay with a cluster threshold of 50 contiguous voxels. C9 = C9orf72 gene expansion-positive group.
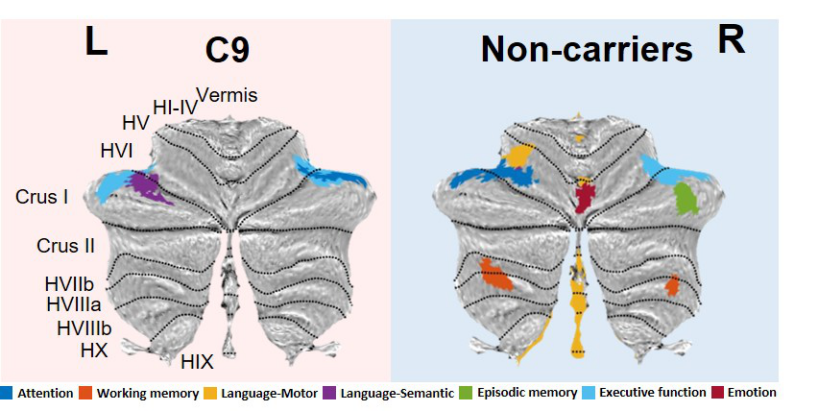
Figure 11. Voxel-based morphometry results showing regions that correlate with cognitive performance after covarying for cerebral atrophy. Coloured voxels show regions that were significant in the analyses at the exploratory threshold of p < .005 (uncorrected), with a cluster threshold of 50 contiguous voxels. C9 = C9orf72 positive group.
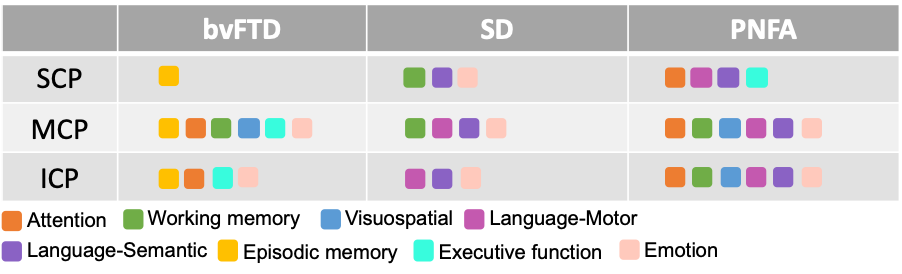
Table 1. Correlations between fractional anisotropy values and neuropsychological performance in each patient groups and Controls combined (p < .05, family-wise error corrected for multiple comparisons). The coloured rounded rectangles indicate the associated cognitive domains. SCP = superior cerebellar peduncle; MCP = middle cerebellar peduncle; ICP = inferior cerebellar peduncle.