Project - Noradrenaline and Cognitive Dysfunction - Interrogating the Neglected Symptoms of Parkinson’s disease
Research Project Abstract
The first stage of our proposal uses a suite of innovative neuroimaging methods in combination with peripheral markers of the noradrenergic system. We apply these tools to a cohort of healthy participants as they undergo functional MRI while performing diverse cognitive tasks. In doing so, Aim I will quantify relationships between ascending sympathetic tone and cognitive function in a cohort of healthy individuals. These experiments will establish mechanistic links between cognitive function, network architecture and the ascending arousal system.
A similar multi-modal imaging approach will then be used to characterize the central and autonomic neural basis of cognitive dysfunction in Parkinson’s disease. Aim II will examine the relationship between cognitive impairment and deficits in autonomic arousal in individuals with Parkinson’s disease. Specifically, we will use multiple autonomic biomarkers to confirm the presence of central noradrenergic deficits across a diverse cohort of individuals with Parkinson’s disease. We will then assess the multimodal neuroimaging signature of cognitive deficits in Parkinson’s disease, testing the hypothesis that an abnormal arousal system predisposes individuals to impaired cognitive function in Parkinson’s disease through a failure to effectively integrate the network architecture of the brain during cognitive tasks.
Project tag with a disease
PD, DLB
Challenges within the field
- Imaging the ascending arousal system, which has a small locus, wide influence and intimate relationships with sources of systemic noise.
- Conceptualising cognitive function in a way that is commensurate with the models we use to understand the impact of the ascending arousal system on the rest of the brain.
Research Project Description
Structural markers of noradrenergic impairment – recent advances in structural imaging have led to the development of novel sequences that can provide structural indices of noradrenergic cell integrity. T1-neuromelanin scans are sensitive to concentrations of neuromelanin, which is particularly high in the noradrenergic locus coeruleus; and magnetic resonance spectroscopy of the pons identifies the strength of chemical signatures of particular neurotransmitters in specific locations in the brain. Previous work has demonstrated a positive link between neuromelanin intensity and whole-brain noradrenergic uptake (2). We predict similar relationships between structural noradrenergic integrity and pupil diameter, both during rest and as a function of task performance. Recent work from other groups (2) suggests that this experiment has a high chance of success.
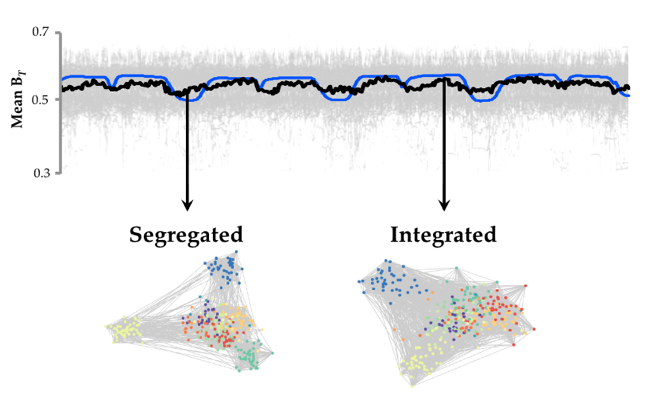
Noradrenergic network signatures during the resting state – We will use our recently devised pipeline for analyzing fluctuations in network structure over time (16) to track alterations in functional network structure while individuals with Parkinson’s disease perform the suite of cognitive tasks. In previous work, we demonstrated that the functional network signature of the brain fluctuates over time between states of high and low ‘integration’ (16). In addition, we documented a relationship between fluctuations in pupil diameter (a measure of central noradrenergic tone) and integration within a network of frontoparietal, striatal and thalamic regions. Given the positive relationship between network structure and pupil diameter, we hypothesize that individuals with a dysfunctional central noradrenergic system (such as those with Parkinson’s disease and cognitive impairment) will demonstrate abnormalities in patterns of network architecture that are sensitive to noradrenergic mechanisms – namely, brain-wide integration. To test this prediction, we will investigate the relationship between network structure and network fluctuations during the ‘resting state’.
Noradrenergic network signatures during performance of cognitive tasks – To determine whether abnormalities in our measures of central sympathetic tone relate to ongoing deficits in cognition, we will investigate fluctuations in network topology during the performance of a suite of cognitively challenging tasks. These tasks will include an ‘N-back’ task (http://www.cognitiveatlas.org/) to investigate working memory and cognitive control; a Stop-Signal task to investigate response inhibition; and a ‘global-local’ task to investigate attentional focus and the ability to avoid distraction. Each of these domains is impaired in Parkinson’s disease (59) and is also to be sensitive to noradrenergic function (60-62).
Research Objectives
Brain mechanisms: There is widespread consensus that identifying the neural processes underlying neurodegenerative disease is pivotal to advancing better treatments, as these processes specify the mechanisms (e.g. direct, psychological, pharmacological) that can be modulated to confer potential benefits to people with the disorders. Indeed, understanding the basics of brain-behaviour processes is currently the Number One strategic priority of the NIMH (Strategic Plan, 2008).
Treatment: Impairments in cognition pervade subjects’ lives and are often highly distressing, leading to high morbidity with nursing home placement (4). If our experiments provide strong support for our hypothesis, we can immediately identify a suite of novel therapeutic targets, including: a) increasing synaptic noradrenaline by blocking reuptake (63); or b) increasing noradrenaline synthesis (64). In addition, the results of Aim 1 may also validate an inexpensive measure for tracking alterations in cognitive function in the clinical setting.
Reverse Translation: There are many ways in which the noradrenergic system can be causally manipulated in animal models (such as the mouse), using tools such as optogenetics to drive the system towards improved or impaired cognitive performance. That power provides opportunities to uncover nuanced ways to improve cognitive function through the enhancement of the interaction between the sympathetic and central nervous systems.
Other disorders: Parkinson’s disease is not the only neurodegenerative disorder associated with pathological involvement of the noradrenergic system. Indeed, pathology of the locus coeruleus may be an indicator of cognitive deficits in Alzheimer’s disease (65). Hence, our work may provide a template for researchers in other areas of clinical neuroscience.