Project - Drug discovery for neurodegenerative diseases
All Chief investigators and associate investigators
Prof. Michael Kassiou
Research Project Abstract
Frontotemporal dementia (FTD) is a neurodegenerative disease that can involve changes in behaviour, social conduct and language. In motor neuron disease, the nerves that control our muscles selectively degenerate, leading to loss of motor control. While these two diseases seem very different, a portion of patients with one of these diseases also show symptoms of the other. In a subset of patients, the two diseases may involve common pathological changes in the brain, such as clumping of a protein called TDP-43, emergence of brain inflammation (neuroinflammation) before symptoms occur and the switching of cells into a senescent phenotype.
The Kassiou Drug Discovery Laboratory is developing imaging agents to detect and limit these common changes. This may allow detection of relevant brain changes before symptoms appear. It may also allow monitoring of the progress of the diseases in clinical trials for new therapeutics. The Lab is also developing new small molecules to correct the TDP-43 aggregation and neuroinflammation seen in these diseases. To increase the chance of translation of these drugs, we are developing new cell models of these diseases using cells derived from patients. We are also developing pro-social small molecules to help with the social symptoms of FTD.
Disease area:
MND, FTD, AD
Challenges within the field
- Development of early diagnostic imaging agents,
- Development of agents to treat and monitor neuroinflammation and
- Development of drug discovery platforms to increase the translatability of neurodegenerative drug discovery
Research Project Description
Our lab seeks to develop novel molecules to treat and detect important hallmarks and symptoms of neurodegenerative diseases like frontotemporal dementia, motor neuron disease and Alzheimer’s disease. We are working towards this across five areas:
- One key change in neurodegenerative diseases is that a subset of proteins start behaving abnormally. These abnormal changes can include losing normal functions, moving into areas of the cells that they don’t normally inhabit, and forming aggregates. We are developing drugs that detect and limit abnormal protein behaviour through techniques such as tissue culture, protein production, western blots and immunofluorescence.
- Another key change in neurodegenerative diseases is the presence of a neuroinflammatory state that appears early in the disease process. We are developing drugs that detect and limit neuroinflammation with a multi-targeted approach using techniques such as iPSC-derivation of glial cells, radioligand binding, cellular assays and western blots.
- As we age, and in a number of neurodegenerative diseases, some glial cells switch into a senescent state. In this state, they lose the ability to replicate and they release deleterious molecules which can contribute to healthy cell loss. We are developing drugs that reduce senescent cell numbers using techniques such as tissue culture, cellular assays, western blots and immunofluorescence.
- Behavioural variant frontotemporal dementia is the most common form of frontotemporal dementia. Patients may demonstrate impaired regulation of socially-appropriate behaviour. We are developing pro-social molecules to assist with the social symptoms of this disease using techniques such as tissue culture, cellular assays and radioligand binding.
- There has been much failure of drug development for neurodegenerative diseases. One reason for this is that the models used to test drugs in the early phases of drug discovery (eg traditional 2D secondary cell line models) don’t fully recapitulate the complex disease environment in the brain. We are developing improved culture models using 3D cultures of iPSC-derived cells.
Research Objectives
- Develop novel drugs that:
- - detect and correct proteinopathy in neurodegenerative diseases
- - detect and correct neuroinflammation
- - reduce senescent glial cells
- - improve the social symptoms of FTD
- Develop high-throughput drug screening platforms that show improved translatability to currently used approaches
Key Publications from this project
- Sokias, R., Werry, E., Cheng, A., Lloyd, J., Sohler, G., Danon, J., Montgomery, A., Du, J., Gao, Q., Hibbs, D., Reekie, T., Kassiou, M., et al (2020). Tricyclic heterocycles display diverse sensitivity to the A147T TSPO polymorphism. European Journal of Medicinal Chemistry, 207, 112725. [More Information]
- Cheng, H., Sokias, R., Werry, E., Ittner, L., Reekie, T., Du, J., Gao, Q., Hibbs, D., Kassiou, M. (2019). First nondiscriminating translocator protein ligands produced from a carbazole scaffold. Journal of Medicinal Chemistry, 62(17), 8235-8248. [More Information]
- Bright, F., Werry, E., Dobson-Stone, C., Piguet, O., Ittner, L., Halliday, G., Hodges, J., Kiernan, M., Loy, C., Kassiou, M., Kril, J. (2019). Neuroinflammation in frontotemporal dementia. Nature Reviews Neurology, 15(9), 540-555. [More Information]
- Qiao, L., Fisher, E., McMurray, L., Sephton, S., Hird, M., Kuzhuppilly-Ramakrishnan, N., Williamson, D., Zhou, X., Werry, E., Kassiou, M., et al (2019). Radiosynthesis of (R,S)-[18F]GE387: A potential PET radiotracer for imaging translocator protein 18 kDa (TSPO) with low binding sensitivity to the human gene polymorphism rs6971. ChemMedChem: chemistry enabling drug discovery, 14(9), 982-993. [More Information]
- Werry, E., Bright, F., Piguet, O., Ittner, L., Halliday, G., Hodges, J., Kiernan, M., Loy, C., Kril, J., Kassiou, M. (2019). Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. International Journal of Molecular Sciences, 20(13), 3161. [More Information]
- Kallinen, A., Boyd, R., Lane, S., Bhalla, R., Mardon, K., Stimson, D., Werry, E., Fulton, R., Connor, M., Kassiou, M. (2019). Synthesis and in vitro evaluation of fluorine-18 benzimidazole sulfones as CB2 PET-radioligands. Organic and Biomolecular Chemistry, 17(20), 5086-5098. [More Information]
- Gulliver, D., Werry, E., Reekie, T., Katte, T., Jorgensen, W., Kassiou, M. (2019). Targeting the oxytocin system: New pharmacotherapeutic approaches. Trends In Pharmacological Sciences, 40(1), 22-37. [More Information]
- Jorgensen, W., Gulliver, D., Katte, T., Werry, E., Reekie, T., Connor, M., Kassiou, M. (2018). Conformationally rigid derivatives of WAY-267,464: Synthesis and pharmacology at the human oxytocin-and vasopressin-1a receptors. European Journal of Medicinal Chemistry, 143, 1644-1656. [More Information]
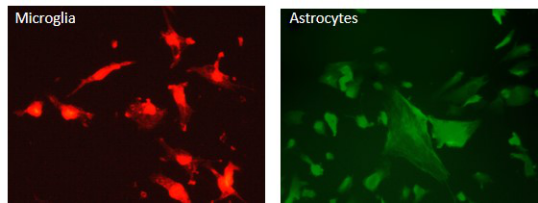
iPSC-derived glial cells
Key Findings
- We have developed an understanding of what drives good binding of ligands to a key protein upregulated in neuroinflammation. Some of the ligands developed from this knowledge have progressed to pre-clinical testing for in vivo detection of neuroinflammation.
- We have developed an understanding of what drives good activity of small molecule brain-permeable ligands to a key pro-social receptor. Some of the ligands developed from this knowledge have progressed to pre-clinical testing, and patents arising from these compounds have been acquired by Kinoxis Therapeutics.
Project related links
Some of our recent publications can be found at the website below: https://sydney.edu.au/health-sciences/about/people/profiles/eryn.werry.php
Information on our FTD research clinic can be accessed here - https://sydney.edu.au/brain-mind/patient-services/forefront-ageing-and-neurodegeneration-clinics/forefront-frontier-clinic-frontotemporal-dementia.html
