Project - Improving the early diagnosis of amyloid- and paraprotein-associated neuropathies: a heterogeneous group with emerging treatments
Disease area:
Neuromuscular: Amyloidosis, Paraproteinaemic neuropathy, Anti-MAG neuropathy, Neurolymphomatosis, CIDP
Research Project Description and Objectives
The aim of this research is to deeply phenotype the amyloid- and paraprotein- associated neuropathies (APAN) in order to determine the key diagnostic features and refine algorithms for diagnosis, thereby improving time to diagnosis and patient outcomes. Specifically, this research will identify the key clinical and imaging characteristics of this disease group, assess for biomarkers of disease progression and disability that can be used to guide and monitor treatment, and prognosticate.
Objectives
- Identify the distinguishing clinical and investigative characteristics (neurophysiology, imaging, biochemical and histological) of APAN.
- Compare the causes of previous misdiagnosis and treatment delay for APAN between regional cohorts.
- Evaluate potential neurophysiological and imaging biomarkers of APAN in pre-symptomatic and symptomatic patients.
- Evaluate potential biochemical biomarkers of APAN in pre-symptomatic and symptomatic patients.
- Develop diagnostic algorithms to guide treatment decisions using the biomarkers identified in Objectives 3 and 4.
The APAN are a heterogenous group of neuropathies that are difficult to identify and frequently misdiagnosed, leading to delays in diagnosis and treatment, accumulated disability and poor quality of life. The pathophysiology of these entities are poorly understood and treatment approaches are often ineffective. Novel treatment options have emerged with the capacity to significantly improve outcomes for patients, however these are reliant on the ability to diagnose APAN at early stages. Moreover, we currently have no validated markers of disease progression. This research aims to define the diagnostic characteristics of the APAN in retrospective and prospective cohorts by deeply phenotyping the individual cases based on the key clinical, neurophysiological, imaging, biochemical and histological features. Prospective evaluation of nerve conduction, axonal excitability, motor unit number estimation and ultrasound will assess for differentiating features and early markers of disease progression. Novel biochemical markers of disease will be assessed for utility in early diagnosis and monitoring. Subsequently diagnostic algorithms will be refined, utilising these methods, with the aim of improving time to diagnosis, aiding treatment decision-making and improving patient outcomes. Further research is essential in order to improve diagnostic and treatment outcomes in APAN.
Specific projects:
- Develop an axonal excitability and motor unit number estimation protocol for use in amyloidosis, where there is frequently a concurrent median neuropathy at the wrist.
- Genotype-phenotype associations in Australian and UK cohorts of hereditary transthyretin amyloidosis (ATTRv)
- Evaluate axonal excitability, motor unit number estimation, small fibre and autonomic studies in normal controls, asymptomatic TTR gene carriers and patients with symptomatic TTR amyloidosis.
- Neurofilament light chain as a biomarker of disease progression in ATTRv
- Neurological phenotypes in wild-type TTR amyloidosis
- Comparative phenotypes in varied haemato-neurological neuropathies.
- Distinguishing inflammatory and haematology associated neuropathies: the role of CSF studies.
- Deep phenotyping to guide treatment in anti-MAG neuropathies
Key Publications from this project
- Carroll, A., Burns, J., Nicholson, G., Kiernan, M., Vucic, S. (2019). Inherited Neuropathies. Seminars in Neurology, 39(5), 620-639
- Carroll AS, Simon NG. Current and future applications of ultrasound imaging in peripheral nerve disorders. World Journal of Radiology. 2020 28;12(6):101.
In submission:
- Sommer. C, Carroll AS, Koike H et al. Nerve biopsy in acquire neuropathies. JPNS
- Carroll AS, Lunn MP. Paraproteinaemic neuropathy: MGUS and Beyond. Practical neurology
- Carroll AS, Doherty CM, Blake J et al. Neurology and the Histiocytoses: a case of Rosai-Dorfman-Destombes disease with widespread neurological manifestations. Practical neurology
- Taylor M, Baumwol, J, Carroll AS, et al. Amyloidosis in 2021. Internal Medicine Journal
Conference proceeding/ Published abstracts:
- AS Carroll, C Doherty, J Blake, et al. Rosai-Dorfman-Destombes disease: Case report with widespread neurological disease including in the peripheral nervous system. JPNS 2020 25 (4), 543544
- AS Carroll, J Howells, C Lin, et al. Axonal excitability in hereditary TTR amyloidosis is consistent with sensory disease burden JPNS 2020 25 (4), 472472
- AS Carroll, A Carr, S D'sa, M Lunn. Episode two: Attack (and reattack) of the clones JPNS 2020 25, s8s8
- JA Bomsztyk, P Jareonsettasin, A Rismani,… AS Carroll et al. Treatment of bing neel syndrome: using a sledgehammer to crack a nut? Hematological Oncology 2019 37, 460460
- JA Bomsztyk, P Jareonsettasin, AS Carroll, et al. Bing neel syndrome: first suspect, then prove‐a role for CSF IgM analysis? Hematological Oncology 2019 37, 46046
- AS Carroll, J Howells, CY Lin et al. Nerve excitability properties of upper limb sensory and motor axons: a comparative study. PNS 2019.
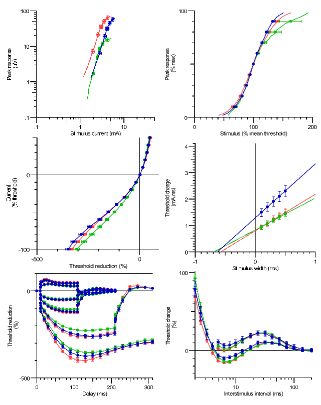
Infographic / Medical Diagram / Scientific Diagram / Picture
Upper limb Sensory peripheral nerve excitability in normative controls. Red: Median, Green: Radial, Blue: Ulnar (A) Peak response curve, (B) Stimulus Response curve, (C) Current Threshold relationship (I/V), (D) Strength Duration relationship, (E) Threshold Electrotonus, (F) Recovery Cycle